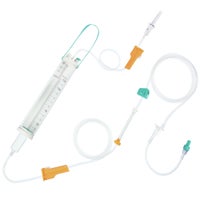
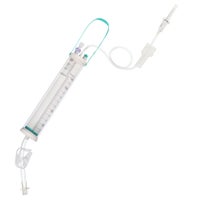
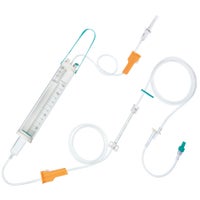
Dosifix® SafeSet
IV administration set with dosing container.
In gravity infusion, Dosifix® SafeSet allows a precise dosage of solution which is given to the patient. This functionality is particularly important for neonatal, pediatric and geriatric care. In gravity or pump-controlled* flow-situations Dosifix® SafeSet is also used for an intermittent infusion of medication in its correct dilution in a closed system with the ability to flush the IV line after each application for mono and multiple therapy without the need to re-spike.
Advantages
Dosing Function:
Fluid overload in critical illness can have harmful effects and contribute to morbidity and mortality.1,2,3 To prevent the accidental over-infusion of large volumes of fluid, intravenous fluids and transfusions should be given with an in-line burette to ensure that the exact doses of fluids prescribed are administered, especially for neonates and paediatric patients.4 The calibrated 150 ml-dosing container of Dosifix® SafeSet allows a precise dosage of solution which is given to the patient and thus reduces the risk of overdosage.5
Admixture Function:
Due to its admixture port on top of the dosage container, Dosifix® SafeSet can be used for an intermittent infusion of medication in its correct dilution with the ability to flush the IV line after each application.5
Flushing Function:
Being connected to an IV container with neutral solution (e.g. NaCl 0.9 %) Dosifix® SafeSet provides the possibility to flush the entire IV line after each drug.5 Flushing helps to prevent the risk of drug incompatibilities when giving multiple doses of intermittent infusions of different drugs and reduces the residual volume of highly effective medication which decreases the risk of therapy failure caused by substance loss.6,7
AirStop:
Dosifix® SafeSet provides the important AirStop function which helps to protect against air infusion. A hydrophilic depth filter membrane inside the drip chamber maintains a constant fluid level when the burette has run empty and prevents the infusion line from running dry.5
PrimeStop:
Lined with a hydrophobic, bacteria-tight membrane, the protective PrimeStop cap of Dosifix® SafeSet stops fluid leaks while priming the set.5
Functional Hanger Design for stabilization of the Dosifix® SafeSet dosing container.5
Highlighted scaling values and optimized swimmer design for visualization of fluid level.5
Closed System:
Being connected to an IV container with neutral solution (e.g. NaCl 0.9 %) and equipped with the needle-free, self-sealing Safeflow port on top of the burette as well as the AirStop filter inside the drip chamber, Dosifix® forms a Closed System acc. to NIOSH. It prevents microbial ingress and the escape of any solution or contaminants into the adjacent environment.5,8
Technical data
- Available with needle-free Safeflow or Safsite ports and needle-based ports.
- Available with Back Check Valve (needle-free set configurations) to prevent back-flow during bolus injection
- Available with Micro (60drops / ml) and Macro (20 drops / ml) Dropper.
- UV-protected set configuration available (REF.No. 4037016).
- * Pressure resistant up to 2 bar. Can be used with non-dedicated infusion pumps, e.g. Infusomat® Space P or Infusomat® compactplus P according to the manufacturer‘s instruction for use of the respective infusion pump.5 Note that article No. 4037016 (Dosifix® with UV protection) can be used in gravity flow situations only.5
- Can be used with luer compatible devices in compliance with DIN EN 1707.5
- Can be used with closures for infusion bottles acc. to DIN EN ISO 8536-2.5
- Tubing diameter: 3.0 x 4.1 mm.5
- Total set length: 225 cm (185 cm below burette)5
- Not made with DEHP.5
- Not made with latex.5
- Dosifix® SafeSet should be changed acc. to national guidelines (e.g. CDC) and / or institutional protocols. In case being used with infusion pumps set must be changed at least 24h.5
- Dosifix® SafeSet can be used for all patients (adults, pediatric and neonates) for which infusion therapy is prescribed. No gender- or age-related limitations.5
1 Ker, G. L., Gangadharan, S. (2018): Management of Fluid Overload in the Pediatric ICU, Pediatric Critical Care, July 2018, pp. 193–209.
2 Foland, J.A., Fortenberry, J.D., Warshaw, B. L. et al. (2004): Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis, Crit Care Med. 2004 Vol. 32, No. 8, pp. 1771–1776.
3 Del Granado, R. C. and Mehta, R. L. (2016): Fluid overload in the ICU: evaluation and management, Nephrology 17, p. 109.
4 WHO (2013): Hospital Care for Children. Guidelines for the Management of Common Childhood Illnesses, 2nd edition. Geneva.
5 Engineering data on file.
6 Plagge, H., Golmich, J., Brnanad, D., et al. (2010): Evaluation of the dead volume in intravenous short-term infusion, Pharma Publishing and Media Europe, Volume 16, Issue 2, pp. 31-37.
7 Federal Institute of Drugs and Medicinal Devices (2015): The Forgotten Residue: Dead Volumes of Short-term Infusions, Pub. No. 2, June 2015.
8 National Institute for Occupational Safety and Health (2004): NIOSH alert 2004-165. Preventing occupational exposures to antineoplastic and other hazardous drugs in health care settings, CDC, Cincinnati, OH.